Contact us for more insights here
Whatever the question, we're here to help.

Trend #1
Increasing adoption of Industry 4.0
Trend #2
Sustainable and environmentally friendly
Trend #3
Digital tools and technologies in supply chain
Trend #4
Personalised and customized products
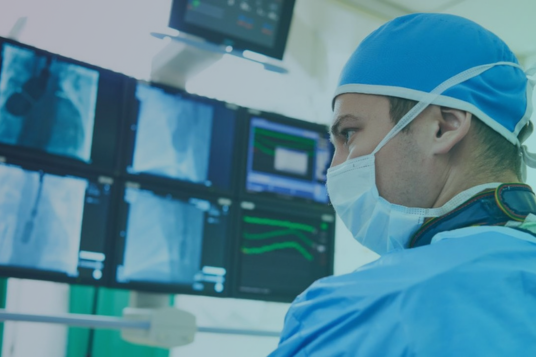




With quality, efficiency, and traceability paramount due to safety issues and recalls, our leading solutions facilitate smart manufacturing in the Pharmaceutical and Medical Device industries.
VIEW ALL CASESAt Emixa, we serve businesses everywhere with technology to help them transform into the future. From SMB's to large OEM's, we support a range of businesses. Explore our downloads to find out how.




Whatever the question, we're here to help.
Discover how the Czech Republic’s largest manufacturer of surgical and dental tools is using Solid Edge CAM Pro to outshine its competitors. In the manufacturing of high-quality implants for traumatology and orthopedics, our solutions help get leading products to market faster.

In the competitive landscape of industrial machinery, the relentless pursuit of innovation is the hallmark of success. For XYZ Corp, a longstanding player in the industry, this pursuit was no less than a mission.
Faced with the challenge of keeping pace with the rapid advancements in technology, they embarked on a transformational journey that would redefine the way they approached manufacturing.

In the competitive landscape of industrial machinery, the relentless pursuit of innovation is the hallmark of success. For XYZ Corp, a longstanding player in the industry, this pursuit was no less than a mission.
Faced with the challenge of keeping pace with the rapid advancements in technology, they embarked on a transformational journey that would redefine the way they approached manufacturing.

Connect with us to revolutionise your medical and pharmaceutical operations.